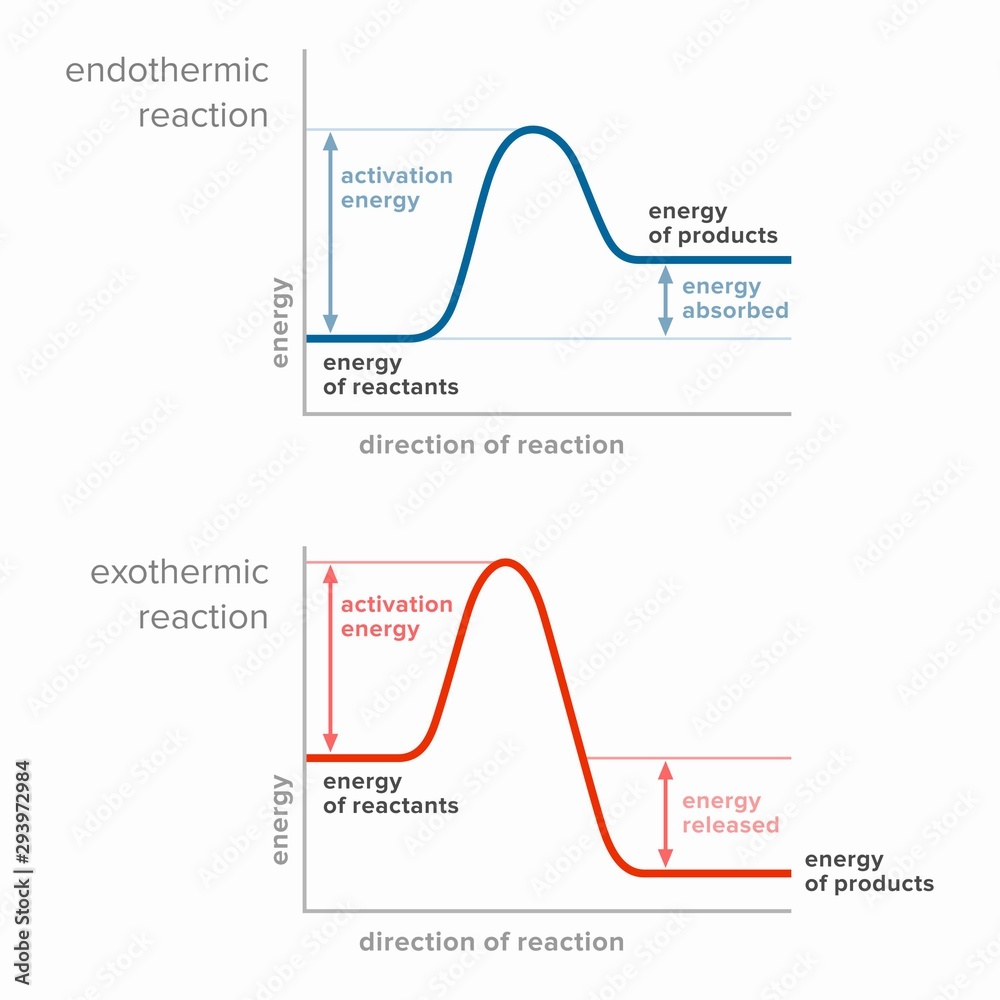
Bond Formation Is Exothermic Or Endothermic
Bond Formation Is Exothermic Or Endothermic - Exothermic reactions, in solution, give out energy and the temperature increases, while. In summary, there are two factors which determine whether a gaseous reaction will be.
Exothermic reactions, in solution, give out energy and the temperature increases, while. In summary, there are two factors which determine whether a gaseous reaction will be.
In summary, there are two factors which determine whether a gaseous reaction will be. Exothermic reactions, in solution, give out energy and the temperature increases, while.
energy Is Bond Formation "Strictly" Exothermic? Chemistry Stack
Exothermic reactions, in solution, give out energy and the temperature increases, while. In summary, there are two factors which determine whether a gaseous reaction will be.
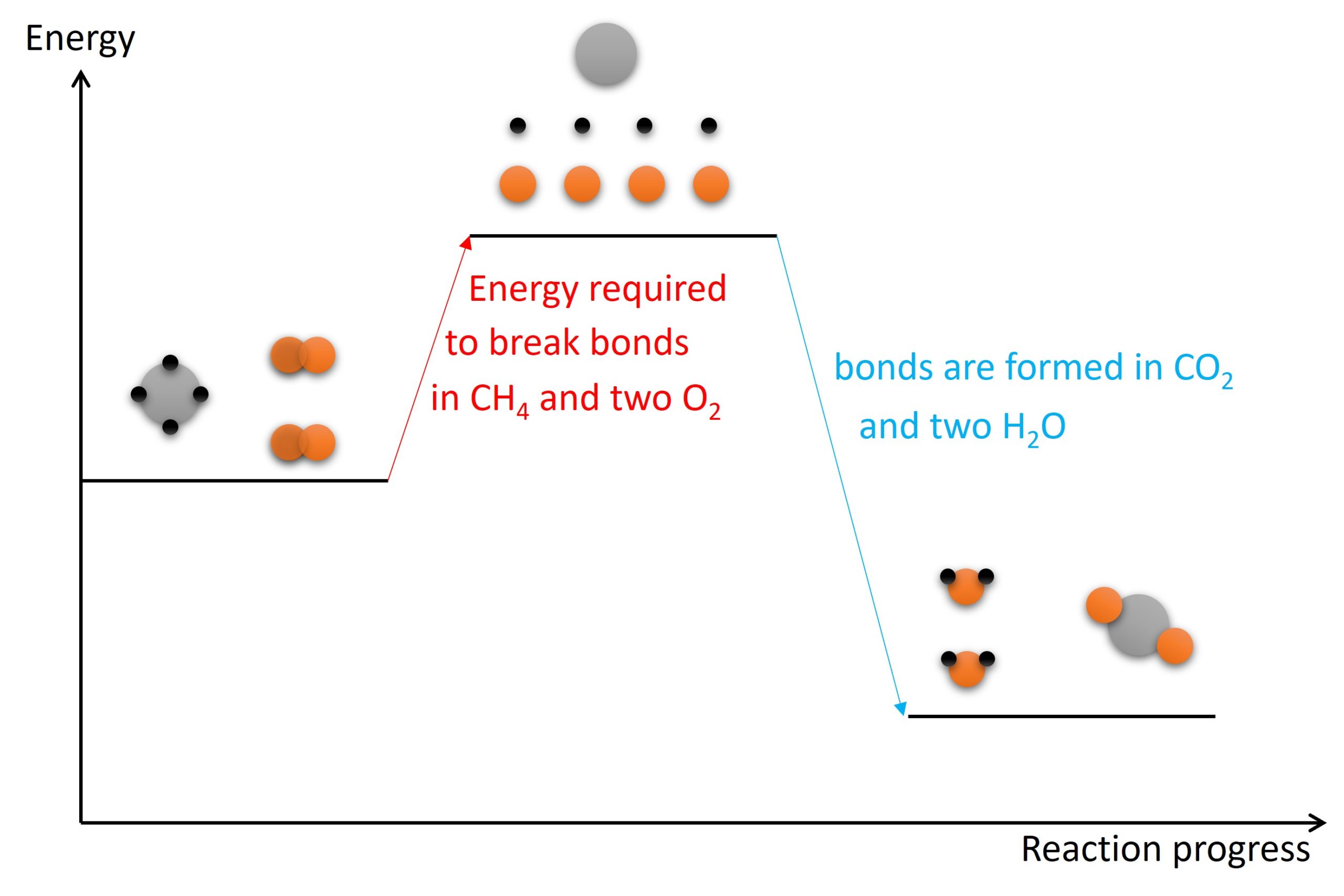
Exothermic and Endothermic reactions, Bond energy, Heat changes
Exothermic reactions, in solution, give out energy and the temperature increases, while. In summary, there are two factors which determine whether a gaseous reaction will be.
B/B Bond Formation exothermic vs Bond cleavage exothermic ATP
In summary, there are two factors which determine whether a gaseous reaction will be. Exothermic reactions, in solution, give out energy and the temperature increases, while.
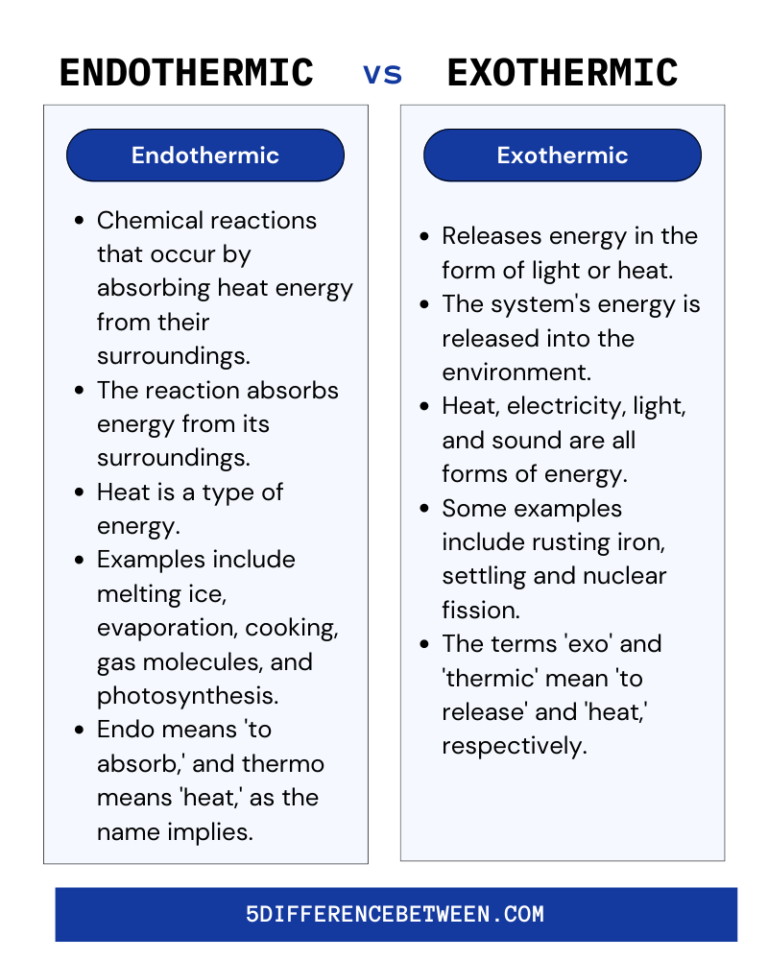
5 Difference Between Endothermic and Exothermic Reaction Endothermic
Exothermic reactions, in solution, give out energy and the temperature increases, while. In summary, there are two factors which determine whether a gaseous reaction will be.
5.1 Exothermic and endothermic reactions IGCSE and A Level Chemistry
In summary, there are two factors which determine whether a gaseous reaction will be. Exothermic reactions, in solution, give out energy and the temperature increases, while.
Solved 1. Bond making is always a. endothermic, exothermic ,
Exothermic reactions, in solution, give out energy and the temperature increases, while. In summary, there are two factors which determine whether a gaseous reaction will be.
Find instructions for performing your own hot and cold chemistry
In summary, there are two factors which determine whether a gaseous reaction will be. Exothermic reactions, in solution, give out energy and the temperature increases, while.
SOLVED Question 11 (2 points) Chemical bondbreaking is a process
Exothermic reactions, in solution, give out energy and the temperature increases, while. In summary, there are two factors which determine whether a gaseous reaction will be.
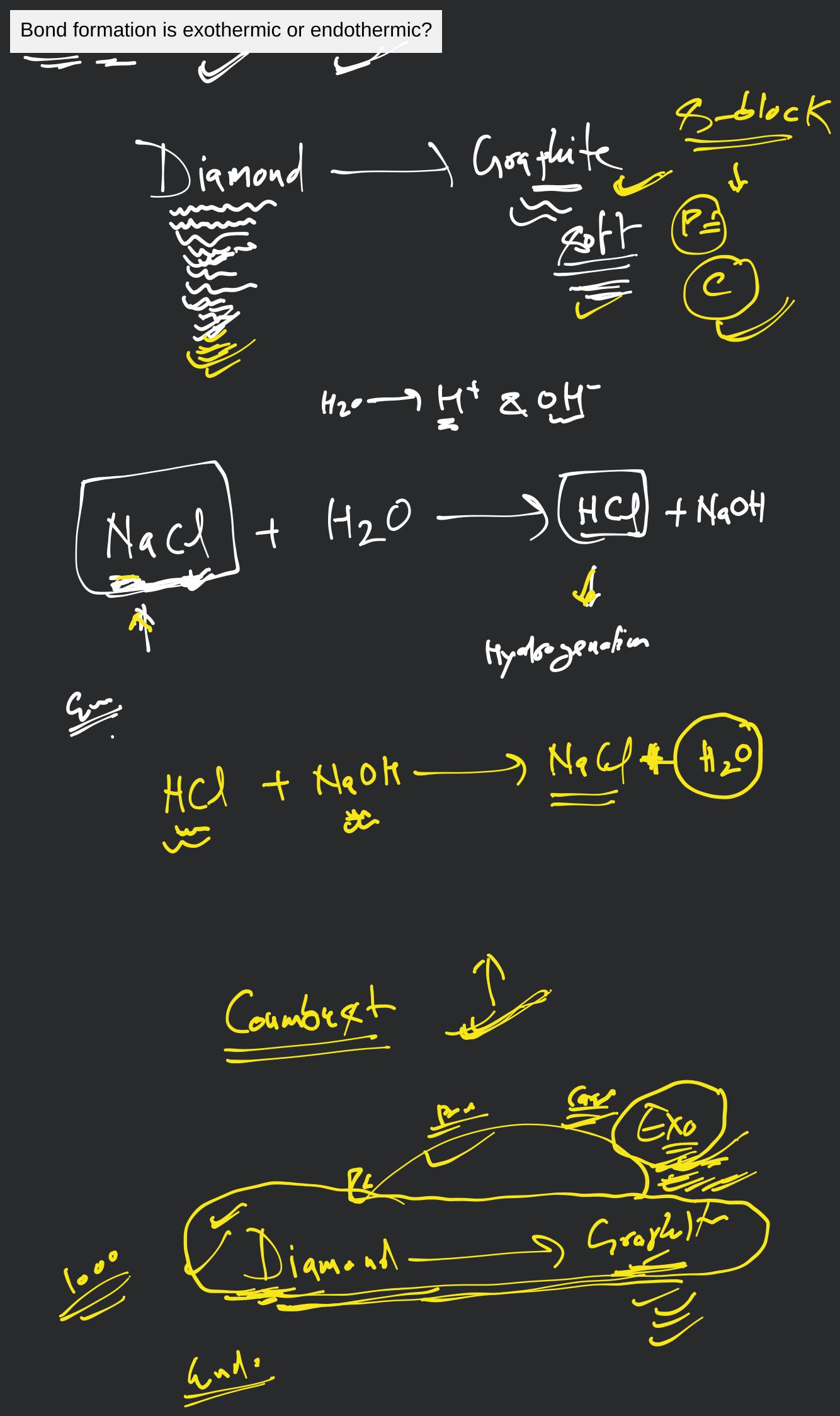
Bond formation is exothermic or endothermic? Filo
In summary, there are two factors which determine whether a gaseous reaction will be. Exothermic reactions, in solution, give out energy and the temperature increases, while.
In Summary, There Are Two Factors Which Determine Whether A Gaseous Reaction Will Be.
Exothermic reactions, in solution, give out energy and the temperature increases, while.